What Makes a Good Buffer Solution
Phosphate buffer mixed Standard buffers are the solutions that have a standard pH. Buffers should not permeate cell membranes.

Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
What are the 4 ways to make a buffer.

. Both solutions must contain the same buffer concentration as the concentration of the buffer in the final solution. When a normal quantity of strong acid or base is introduced to it the pH hardly changes. Read customer reviews find best sellers.
What makes a good buffer in chemistry. Many natural systems rely on buffering to maintain pH balance. Goods buffers are twenty buffering agents for biochemical and biological research selected and described by Norman Good and colleagues during 19661980.
For example if 1221 grams of solid sodium benzoate are dissolved in 100 L 0100 M benzoic acid C 6 H 5 COOH pK a 419 solution a buffer with a pH of 419 will result. Adding a strong base to a weak acid. Adding a conjugate base to a weak acid.
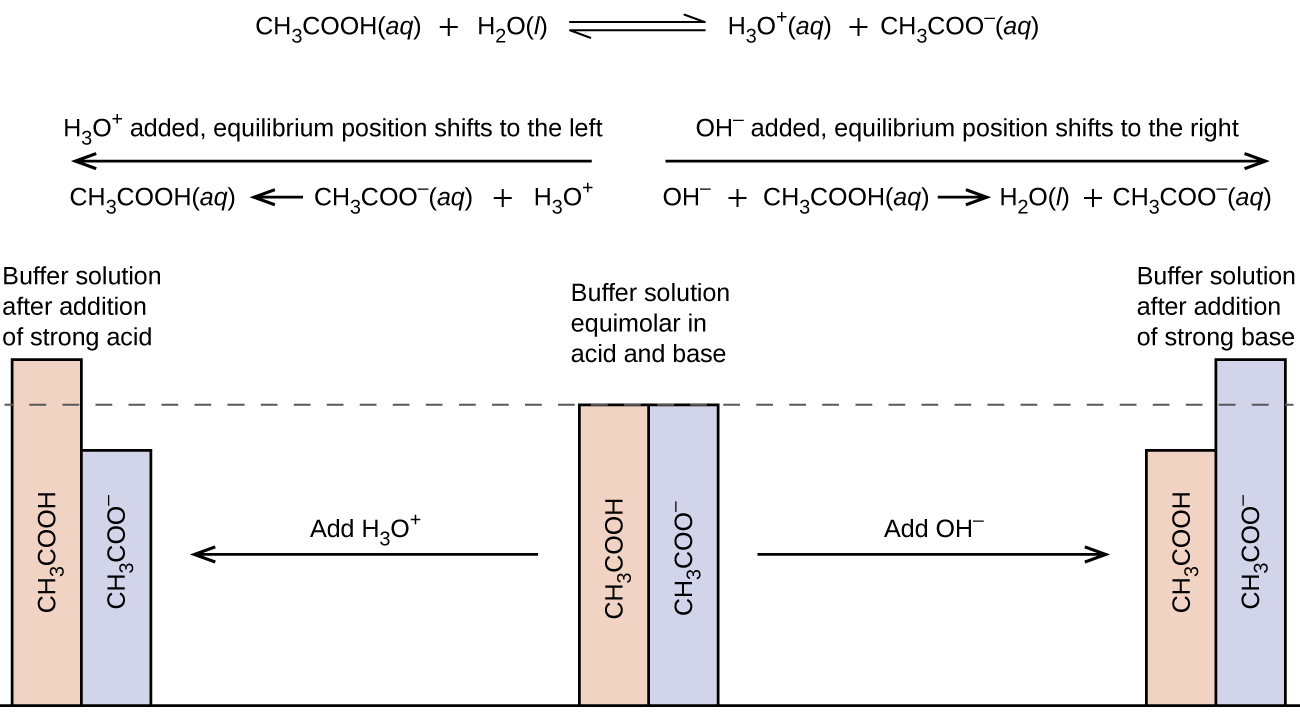
Before Goods work few. A buffer solution is an aqueous mixture of a weak acid and its conjugate base. Buffer solutions are widely used in chemical applications to keep pH at a constant value.
A buffer is an aqueous solution consisting of a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. A buffers pH changes very little when a small amount of strong acid or base is added to it. Most of the buffers were new zwitterionic compounds prepared and tested by Good and coworkers for the first time though some were known compounds previously overlooked by biologists.
Visit our website for the notes of this lecture. To get the final buffer add one solution to the other while monitoring the pH. There are several ways a solution containing these two components can be made.
D is ok as the NaOH will half neutralised the acid leaving a solution containing the weak acid and the salt of that weak acid sodium acetate. You need weak components for the buffer to work. Buffers should have high water solubility and minimum solubility in organic solvents so it remains in the aqueous medium of the biological system.
Adding a strong acid to a weak base. For example a mixture of ammonium chloride and ammonium hydroxide acts as a buffer solution with a pH of about 925. A buffer must contain a weak acid and its conjugate base.
In a third method you can determine the exact amount of acid and conjugate base needed to make a buffer of a certain pH using the Henderson. A buffer solution is one in which the pH of the solution is resistant to small additions of either a strong acid or strong base. They are used as reference standards for the measurement of pH.
Buffers should be very soluble in water and minimally so in nonpolar solvents fats oils and organic solvents. Also what makes a good buffer solution. A buffer is either a weak acid and the salt of that weak acid or a weak base and the salt of that weak base.
Good buffers have a high solubility in water since most biological systems naturally use water as their solvent. It is therefore used to prevent change in the pH of a solution upon addition of another acid or base. Also the solubility level of Good buffers in organic solvents such as fats and oils is low.
Buffers usually consist of a weak acid and its conjugate base in relatively equal and large quantities. Buffers should have a pKa between 60 and 80 because the optimal pH for most biological reactions rests in this range. This prevents the buffer from accumulating in cell membranes vesicles and other nonpolar compartments in biological systems.
Palladium chloride buffered solution. Buffers can be made from weak acids or base and their salts. What makes a good buffer solution.
Should have the high chemical stability Low cell membrane permeability Low metal chelating capacity Should have consistent acid-base dissociation constant Low absorption spectra in UV and visible regions. Ad Browse discover thousands of brands. Buffered Copper sulfate solution.

Methods For Preparing Buffers Video Khan Academy
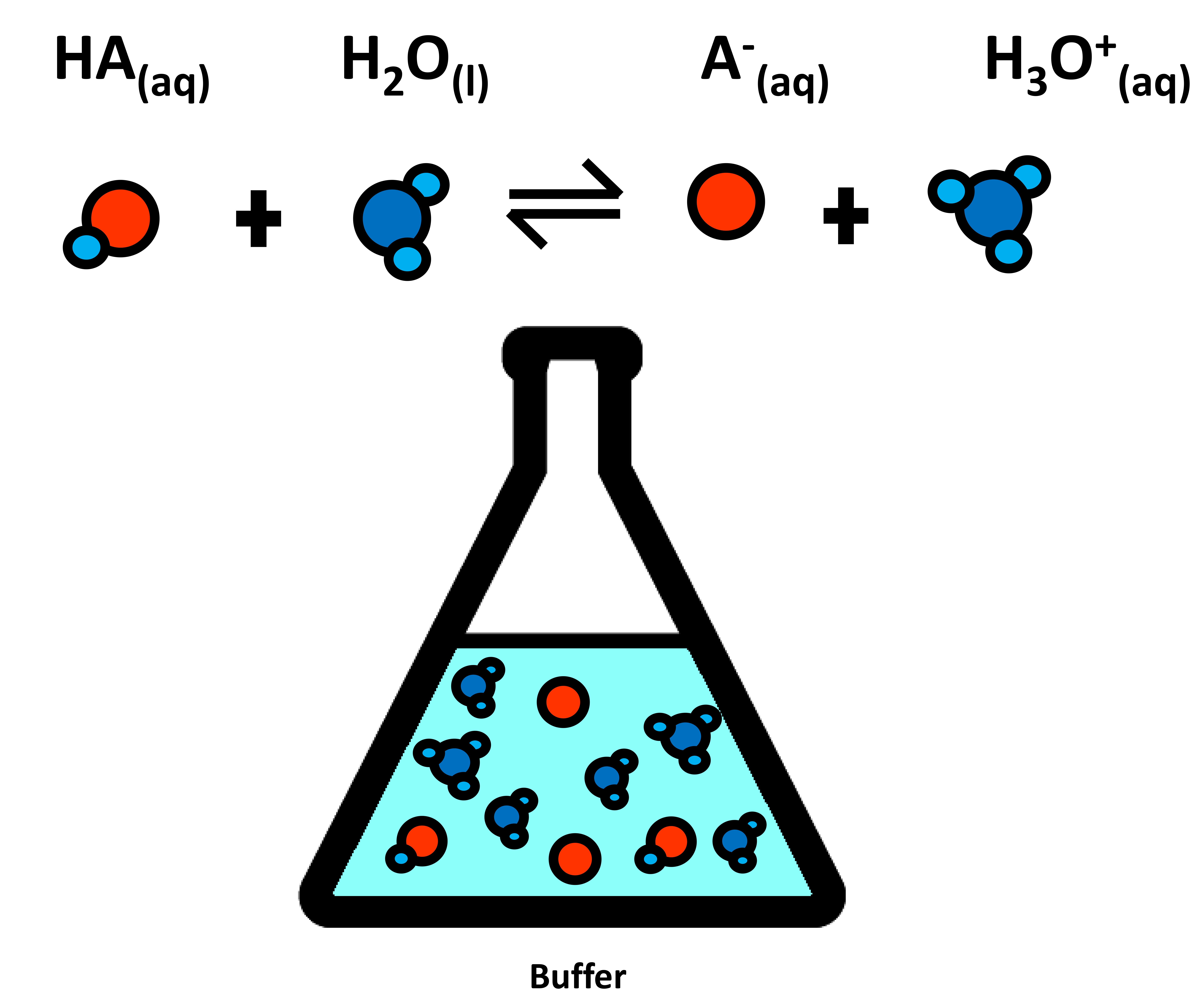
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio

Making Buffer Solutions Osmosis
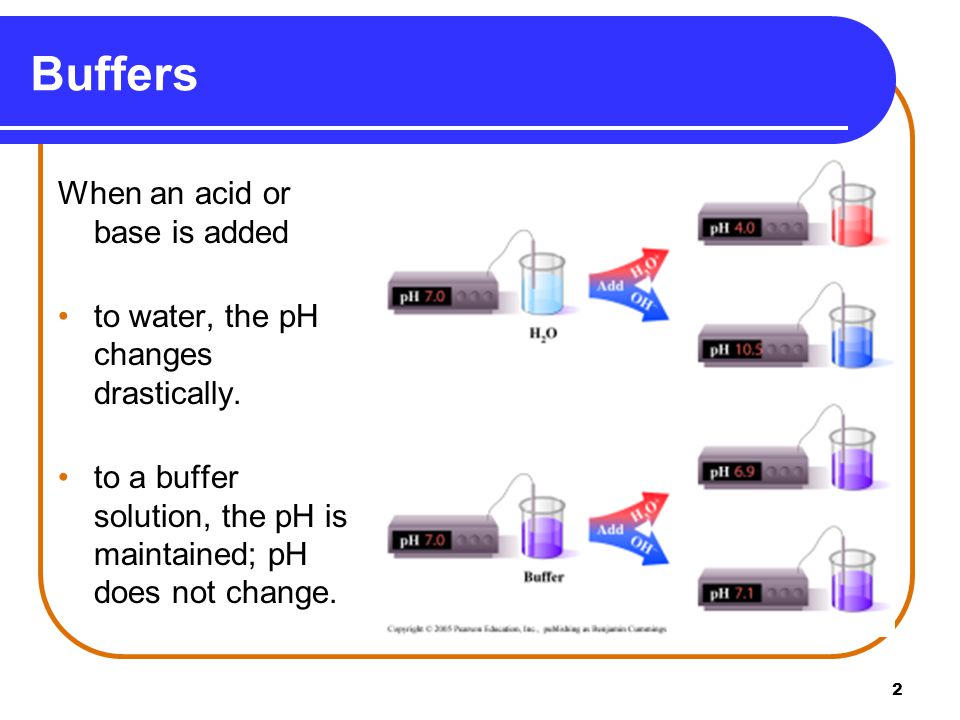
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
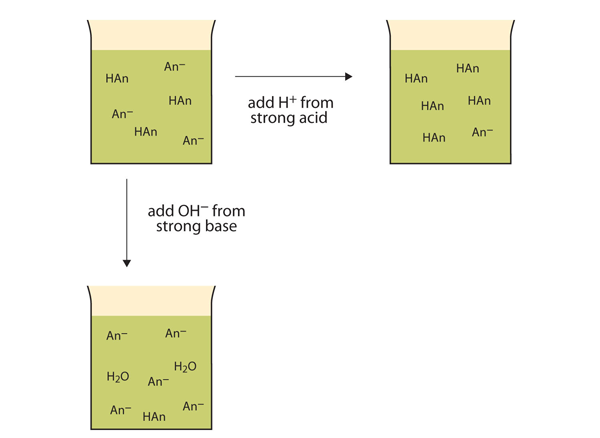
Buffers Introductory Chemistry 1st Canadian Edition
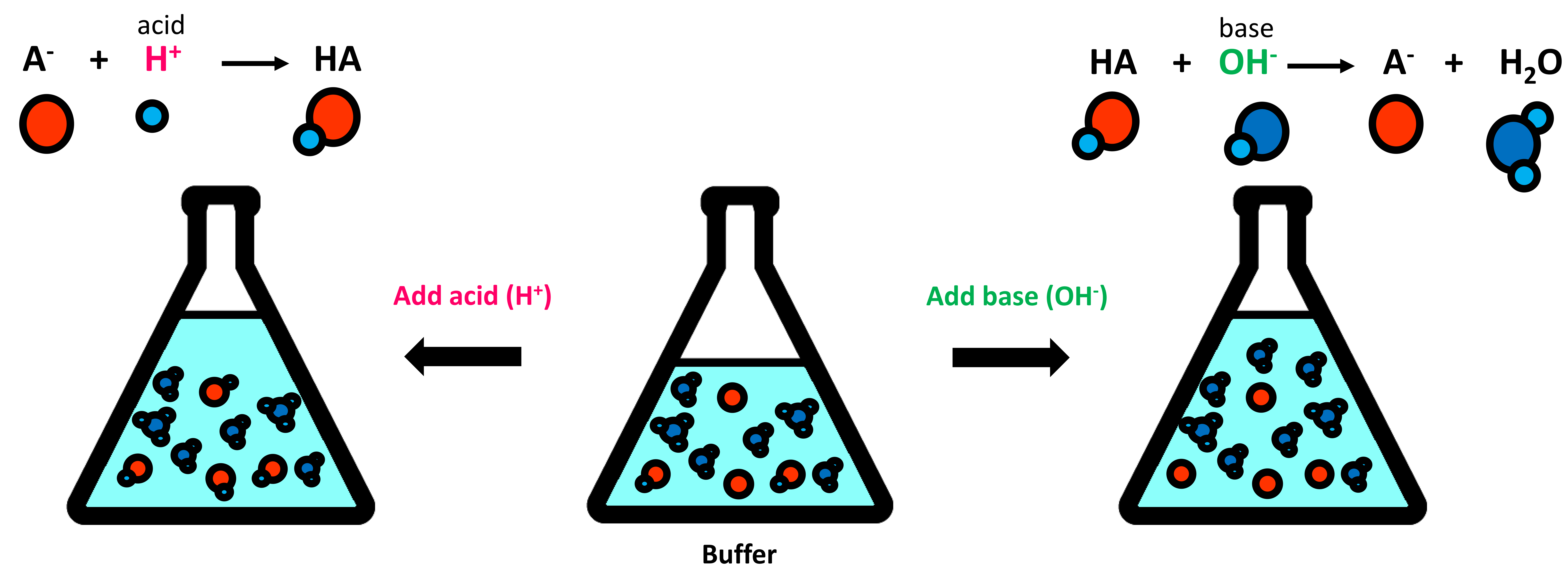
What Is A Biological Buffer And How To Choose The Best Buffer For Your Experiment Goldbio

Tips For Iding If A Solution Is A Buffer Concept Chemistry Video By Brightstorm

Comments
Post a Comment